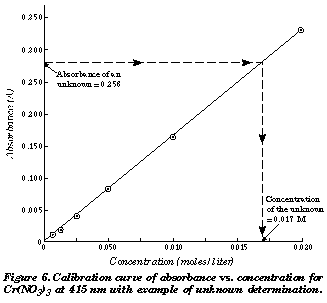
The pulse oximeter computer takes these things factors and computes the saturation. We have a value for y Absorbance and need to solve for x Concentration.
Surface Finishing Tutorial Technical Tutorial Misumi
Difference Between Absorbance And Transmittance
Experiment 1 Cellbiologyolm
Understand the Beer-Lambert law for absorbance A ɛ x l x c.
Light absorbance and concentration graph. The standard equation for absorbance is A ɛ x l x c where A is the amount of light absorbed by the sample for a given wavelength ɛ is the molar absorptivity l is the distance that the light travels through the solution and c is the concentration of the absorbing species per unit volume. Oxyhemoglobin and deoxyhemoglobin absorbs red and infrared light differently. When the data follow a uniform interval on the x-axis eg a times series or equal increments of concentration use major ticks to match the data.
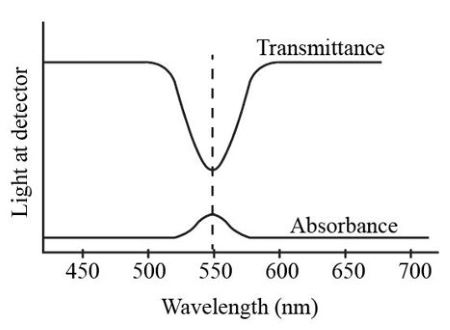
The computer-interfaced colorimeter monitors the light received by the photocell as either an absorbance or a percent. Absorbance is defined as the amount of light absorbed by the molecules of the object. Spectral graph correlates visible wavelengths with color.
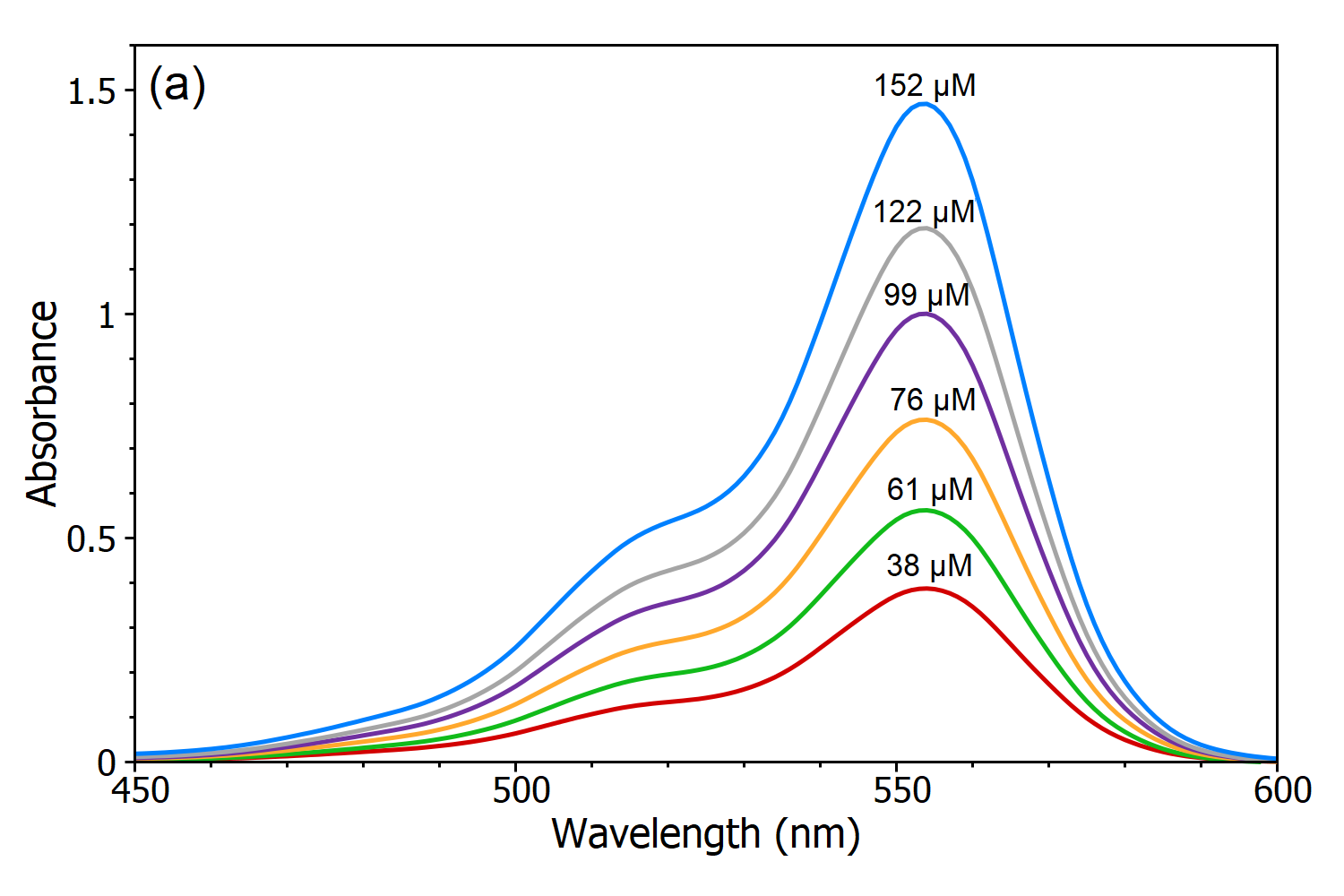
Figure 31 shows that absorbance increases with increased addition of carbon nanotubes because they play a role of radiation screener. Advanced Placement Chemistry. Concentration of the light absorbing substance.
For any given compound the amount of light absorbed depends upon a the concentration b the path length c the wavelength and d the solvent. The instrument used in ultravioletvisible spectroscopy is called a UVVis spectrophotometerIt measures the intensity of light after passing through a sample and compares it to the intensity of light before it passes through the sample The ratio is called the transmittance and is usually expressed as a percentage TThe absorbance is based on the transmittance. Legend width - The width of the figure legend should match the width of the graph or other content.
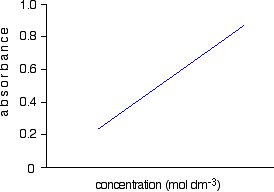
But when making a calibration graph you are looking at the absorbance based on concentration. Frequently the absorbance A rather. Calculate the concentration of lead in the original undiluted sample.
Weigh the applicable amount of ACS-grade sodium chloride. In other words the more material in the solution absorbs the light the less light will get through. Concentration of a chemical based on its absorbance of light.
Remember that absorbance is 1transmittance the spec is really reading how much light goes through the sample. The Nanodrop does an excellent job at measuring across a wide spectrum that spans UV and visible light. Absorbance Beers Law Change Over Time.
Chloride concentration of samples in gL x 16485 g of NaCl per liter. The equation displayed on the chart gives a means for calculating the absorbance and therefore concentration of the unknown samples. ε is the extinction coefficient also referred to as the.
Beers law says that the relationship between the absorbance of the chromophore and its concentration is linear allowing construction of a standard curve by plotting absorbance versus concentration such as shown in Figure 1. Wavelength molar absorptivity constant and the calibration plot is vs. Aebc where ε is the extinction coefficient M-1cm-1 b is the solution path length cm and c is the.
Lastly C is the molar concentration of the chromophore molL used for the measurement. Transmittance is also expressed as a percentage IIo x 100. It should be noted that the absorbance of nanotubes at concentration of 008 wt the highest concentration on the graph was.
Concentration of the absorbing compound in solution. A ε l c Equation 1 A is the absorbance observed. If Io is the intensity of light at 550 nm entering the solution and I is the intensity of light exiting the solution then the transmittance T of the solution is given as IIo.
It cant automatically determine for you that the sample on the pedestal is DNA RNA or protein you have to tell the software before beginning measurements so it. We have been given the absorbance readings for two solutions of unknown concentration. It is likely that the unknown will have absorbance numbers outside the range of the standard.
Using the linear equation labeled A in Figure 5 a spreadsheet cell can have an equation associated with it to do the calculation for us. The two plots above are an Absorbance spectrum on the left and a calibration plot on the left. Where A absorbance c sample concentration in molesliter l length of light path through the sample in cm If the isoprene spectrum on the right was obtained from a dilute hexane solution c 4 10 -5 moles per liter in a 1 cm sample cuvette a simple calculation using the above formula indicates a molar absorptivity of 20000 at the maximum absorption wavelength.
T 010 and A is called the absorbance. Students use a spectrometer to learn how to use visible light to determine the concentration of colored ion species in a solution. Absorbance is related to the concentration according to the Beer-Lambert law.
The absorbance of the sample lies between the absorbance for standards 1 and 2 therefore the concentration of lead in the sample must be between 100 and 200 ppm Step 3. Prepare 1 liter of chloride water that has the same chloride concentration as the samples. Transmittance is a ratio of the incident intensity of light I 0 to the amount of intensity passes through the object I.
Both are plotting absorbance but the spectrum plots it vs. When the absorbance is very high 17 25 depending on the instrument you should look close at your data. Spectrophotometers can be used to determine the amount of a substance in solution by measuring the amount of light transmitted or absorbed as light of a specific wavelength passes through the specimen.
188 gL is the typical chloride concentration of seawater. Therefore a high absorbance means very little light is making it through the sample. Absorbance is defined as.
This relationship is expressed by Beers Law Equation 1. Concentration of lead in the diluted sample was 160 ppm Check that your answer is sensible. Interpreting a Standard Curve A spectrophotometer measures light quantity.
Consequently the absorbance can also be given in terms of the percentage transmittance. This relationship is best determined using a wavelength of light in a region of the visible spectrum where the maximum absorbance is observed. Higher concentration of the colored solution absorbs more blue light and transmits less blue light than a solution of lower concentration.
In Graph 1 x is concentration and y is absorbance so one must rearrange the equation to solve for x and enter the absorbance of the measured unknown. C is the concentration of the analyte b is the length of the light path through the absorbing solution and a is the absorbtivity a number which depends both on the nature of the light absorbing substance and the wavelength of light. Length of the light path in the absorbing substance.
Is that the absorbance is too high. Add the sodium chloride to a 1-liter. No minor intervals would be used in this case.
This wavelength is known as λ max and is most sensitive to the changes in concentration. To summarize things so far the absorbance of light depends on. Relationship between the concentration of the compound being studied and its absorbance.
According to Beer-Lambert law the absorbance of light as it passes through a solution is directly proportional to the path length of light. It tells you how much light is passing through a solution transmittance or how much light is being absorbed by a solution absorbanceIf you graph absorbance versus concentration for a series of known solutions the line or standard curve which fits to your points can be used to figure out the concentrations of an. Equation How the value change as the concentration is increased.
Absorbance of 550 nm light.

A 8 6 Find The Concentration Of A Solution Via Calibration Curve Beer Lambert Law Ib Chemistry Hl Youtube
Beer S Law Theoretical Principles
Using Uv Visible Absorption Spectra
How Do You Calculate Concentration From Absorbance Socratic

1 2 Beer S Law Chemistry Libretexts
Beer Lambert Law Transmittance Absorbance Edinburgh Instruments
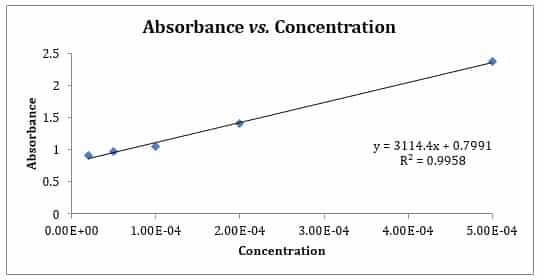
Beer S Law Lab Explained Absorbance Vs Concentration Schoolworkhelper

Plot An Absorbance Vs Concentration And T Vs Concentration In One Graph The Absorbance Values Are So Small Compare To The T Values Study Com

