Lambda max is the wavelength in the absorption spectrum where absorbance hits maximum. The path length refers to the length of sample the light has to go through.
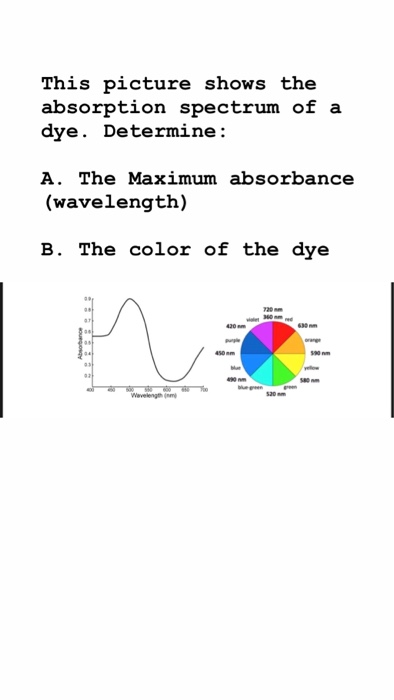
Solved This Picture Shows The Absorption Spectrum Of A Dye Chegg Com

What Is The Effect Of The Wavelength On The Spectrophotometric Measurements Of Protein Biology Stack Exchange

Resolution And Stray Light Uv Vis Spectroscopy Faq
This wavelength will be used to monitor the disappearance of crystal violet throughout the course of the experiment.
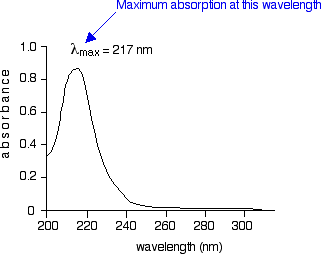
How to determine wavelength of maximum absorbance. Stray Light Recommended Reference Material T at 198 nm reported 190 210 nm Aqueous potassium chloride. The extinction coefficient is a constant specific for an absorbing substance and a specific wavelength typically the absorbance maximum of the substance. Short answerUsing the maximum wavelength gives us the best results.
Use this graph to determine the unknown concentrations of two samples of the protein showing your work. Fill the sample pump or Superloop with the protein sample and inject the sample to the system by-passing the column until the UV absorbance from the sample reaches a. Integrated absorbance not peak absorbance depends linearly on concentration.
Looking at the graph that measures absorbance and wavelength an isosbestic point can also be observed. Set the UV monitor to an appropriate wavelength for your molecule. Potassium persulfate has a strong absorption peak at 220nm which coincides with the absorption wavelength of total nitrogen.
Gives 0000 absorbance based on the absorbance at 275 nm of protein sample. In some cases in order to clearly show weak features spectra were recorded at levels where the maximum absorbance was high. Apply the Beer-Lambert law and absorbance measurements to determine concentration of a coloured species in solution.
Other standard approaches may mix the standard into the unknown giving. Measure absorbance of solutions at the specified wavelength. Here we use 310nm as the wavelength for absorbance.
Determine the wavelength colour of light to use for the colorimetric analysis. Particulate matter can affect the UV-Vis absorbance by scattering incoming radiation. The first step in this analysis will be to determine the wavelength of maximum absorbance for crystal violet and the corresponding molar absorption coefficient at that wavelength.
Atomic absorption methods measure the amount of energy in the form of photons of light and thus a change in the wavelength absorbed by the sample. For example wavelength of 10 micrometer is 1000010 1000 cm-1. Construct and use calibration curves to determine the concentration of an analyte in the analytical solution.
Called the wavelength of maximum absorbance which is abbreviated to λ max spoken as lambda max. This provides maximum sensitivity for your measurements. Choosing Your Wavelength.
Specifically a detector measures the wavelengths of light transmitted by the sample the after wavelengths and compares them to the wavelengths which originally passed through the sample the before wavelengths. A peak of the absorbance curve a wavelength where the absorbance reaches a maximum is where the rate of change in absorbance with wavelength is smallest. But there will be residual potassium persulfate in the solution to affect the absorbance if the temperature-controlled fail to meet the requirement.
The optimal wavelength Amax for measuring absorbance is that wavelength that is most absorbed by the compound in question. For example p-nitrophenol acid form has the maximum absorbance at approximately 320 nm and p-nitrophenolate basic form absorb best at 400nm as shown in Figure 3. For instance in a cuvette the path is standardized to 1 cm.
Based on the preliminary studies we confirmed that at this wavelength maximum aggregation occurs ie maximum absorbance. This is because at the peak absorbance the absobance strength of light will be. Absorbance of two different compounds.
This wavelength is known as λ max and is most sensitive to the changes in concentration. This measurement helps biochemists determine important safety information about substances you may consume and how they may affect your body. In analytical chemistry a calibration curve also known as a standard curve is a general method for determining the concentration of a substance in an unknown sample by comparing the unknown to a set of standard samples of known concentration.
An absorbance spectrum a plot of absorbance as a function of wavelength is determined to select the optimal wavelength for analyzing a given compound. A single point measurement recorded at λ max will be used to calculate the concentration of red yellow green and blue food dyes in a solution. Look at the images above.
Scattering by suspended material in the sample increases with particle size with maxim scattering for a given weight of sample occurring at a particle size equal to the wavelength of incoming radiation. And we can determine the absorbance and concentration with this solution. Prepare a set of standard solutions of known concentration.
The purpose of this experiment was to study the absorbance of CuSO 4. Because copper compounds tend to be blue in color3 it was hypothesized that the CuSO 4 would have a λ max with a longer wavelength. The numerical integration of the absorbance leads to maximum deviations from linearity of less than 01.
Using the FT-IR spectrometer the spectrum is already expressed in the wavenumber. You do not need to do anything. Repeat steps 5-11 for the remaining.
Measure the absorbance of the cut-off solution filters specified against a 10-mm cell filled with an appropriate reference and record the maximum absorbance value A or the minimum Transmittance T at the recommended wavelength. Measurements are usually made at a peak to minimize errors produced by errors in wavelength in the instrument that is errors due to having a different extinction coefficient than assumed. You may repeat.
You will be able to determine which chemical dye was used in the solution. If the isoprene spectrum on the right was obtained from a dilute hexane solution c 4 10-5 moles per liter in a 1 cm sample cuvette a simple calculation using the above formula indicates a molar absorptivity of 20000 at the maximum absorption wavelength. A calibration curve is one approach to the problem of instrument calibration.
If the absorbance reading is not between 05 and 08 then dilute the sample as mention previously until an absorbance reading that falls within acceptable range is obtained. Plot the absorbance vs concentration for each standard solution on a graph. Indeed the entire vertical absorbance scale may be changed to a molar absorptivity scale once this information about the sample is in.
Hover the cursor over the maximum absorbance peak and record the corresponding wavelength x-axis and absorbance y-axis. Measure the absorbance of each standard solution using the colorimeter. The wavelength 410nm was a very good choice for the calibration plot but how do you know which wavelengt is the best wavelength based on the absorbance spectrum.
Print your graph once it is titled Y vs X includes the equation of the line and the R2 value has a label for each axis and includes any appropriate units. Typically this will be 280 nm for proteins and 260 nm for DNARNA. The calibration plot is like taking a vertical slice through the all the absorbance spectra at the specific wavelength 410nm.
Draw the line of best fit through the data points. Prime the system with binding buffer. Maximum absorbance is observed.
Under these conditions relative absorbances of major bands become less uncertain since accuracy depends on the distance between the band maximum and the maximum absorbance limit which can become very small. The concentration dependence of absorbance can deviate from linearity even in the absence of any interactions or instrumental nonlinearities.

Uv Vis Maximum Absorption Wavelength L Max In Nm And Oscillator Download Scientific Diagram

5 Colorimetric Analysis 4 Hitachi High Tech Global
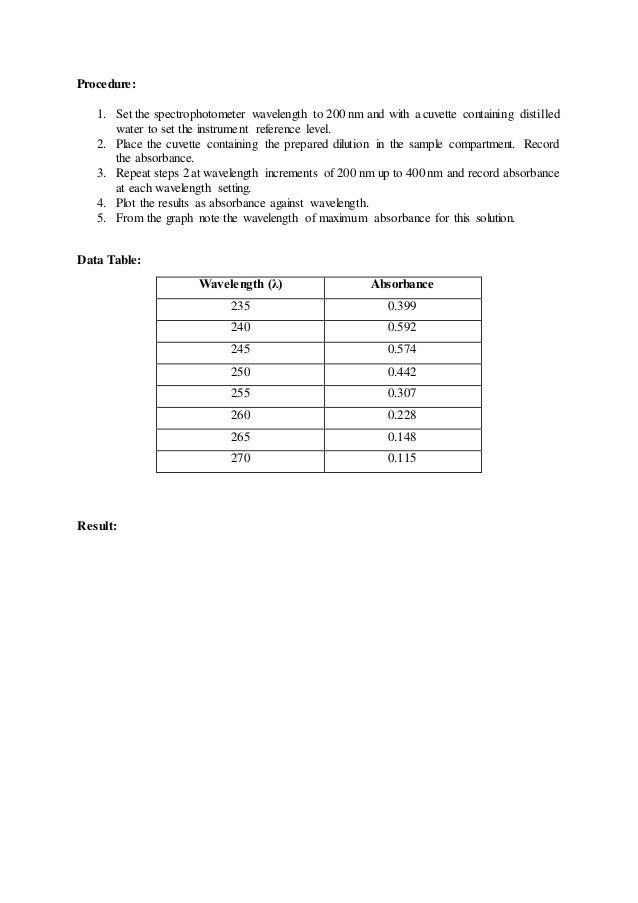
Determination Of A Wavelength Of Maximum Absorbance Lmax And

930 Questions With Answers In Absorption Science Topic

Spectrophotometric Analysis Of The Chromium Ion

Uv Visible Absorption Spectra

Absorbance Measurements Bmg Labtech

Determination Of Wavelength Of Maximum Absorbance

