So if the peak absorbance is below 10 then using the peak wavelength is best but if the peak absorbance is well above 10 you might be better off using another wavelength where the absorbance. Multiply l by c and then divide A by the product to solve for molar absorptivity.
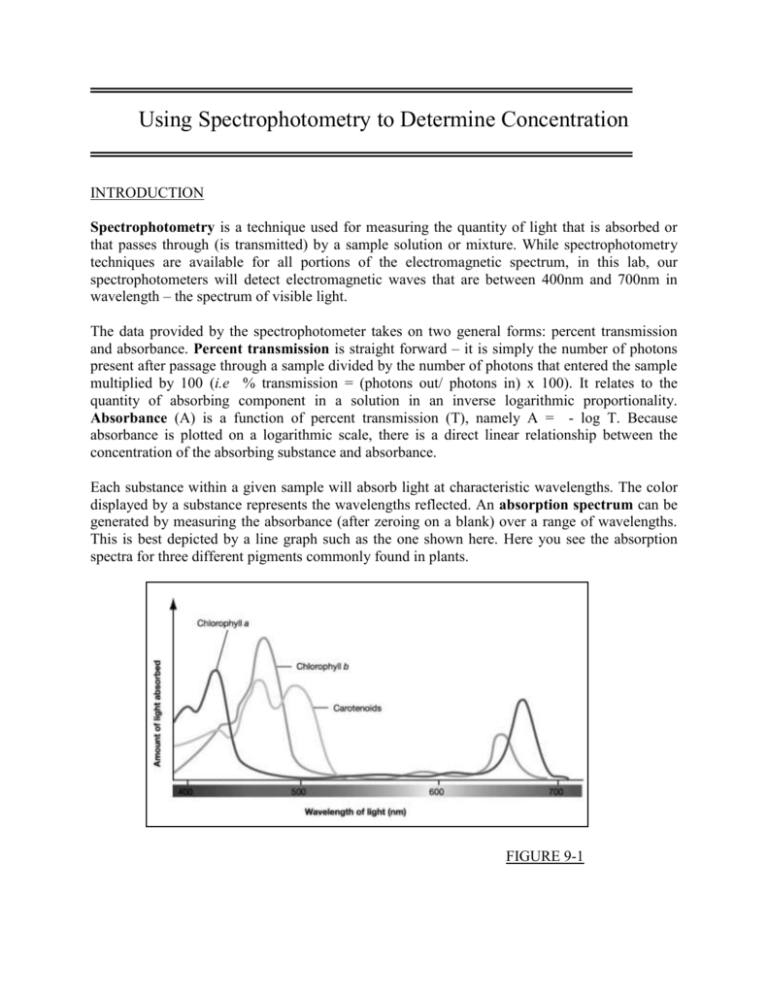
Using Spectrophotometry To Determine Concentration
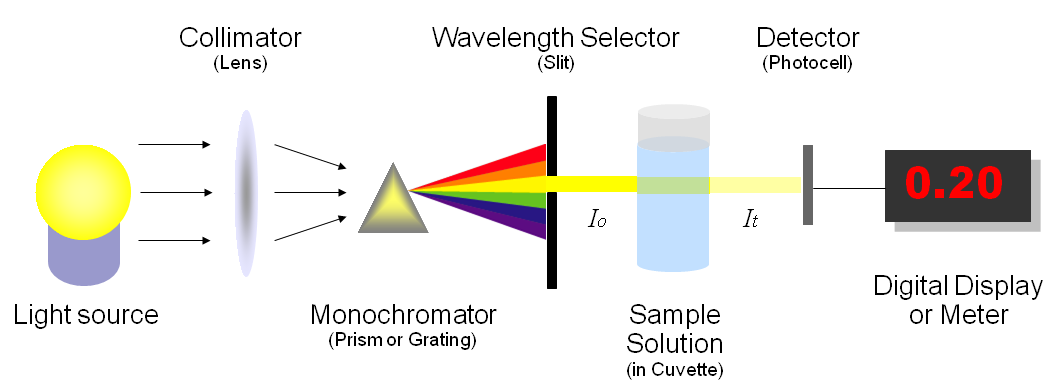
2 1 5 Spectrophotometry Chemistry Libretexts
Experiment 1 Cellbiologyolm
We then measure the absorbance of each diluted solution at the analytical wavelength.

Using absorbance to determine the wavelength. Make sure to check the settings and use the fixed wavelength. Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. The first experiment yielded wavelength and absorbance OD readings as shown in Table 1.
A graph of absorbance vs concentration is called a Beers Law curve in honor of the chemist who first discovered the relationship between absorbance and concentration. Mgml Abs 00051 2 Aspirin in sample 18895 The aspirin content of each tablet is then determined using equation 3. In the case of nucleic acids and proteins for example this would be 260 nm and 280 nm respectively.
The food dye Red 40 has a molar absorptivity of 25900 L mol-1 cm-1 at a wavelength of 501 nm. If the absorbance reading is not between 05 and 08 then dilute the sample as mention previously until an absorbance reading that falls within acceptable range is obtained. A linear regression of the standard absorbance data gave equation 1.
In order to determine the concentration of an analyte most often the wavelength is utilized at which the molecule displays the highest absorbance peak wavelength. Use the sipper or using individual cuvettes. DNA and RNA absorbance measurements using SpectraMax Microplate Readers.
The measured absorbance is 017. 1 y 18895x - 00051 Re-arranging equation 1 allows the aspirin content of the sample solution to be determined according to equation 2. Beers Law allows calculation of the concentration of crystal violet using the experimentally determined value of ε.
If any of these criteria do not apply there is the possibility to indirectly measure analytes andor using a. The purpose of this experiment was to study the absorbance of CuSO 4. Use a spreadsheet program or a graphing calculator to plot your data and determine a best-fit line trend line to calculate the slope of your line.
You place 1 mL of the solution in a cuvette with a width of 1 cm. These results were plotted in Figure 1. To do this we prepare a set of dilutions of the standard dye its concentration is written on the bottle.
From this point the. Where I 0 is the intensity of the incident light and I is intensity of that light after it passed through the sample. Determine the absorbance at 595 VIS lamp.
Control of Absorbance Recommended Reference Material Potassium dichromate solutions 235 nm. Absorbance quantitation of DNA works on samples ranging from about 025 µgmL to about 125 µgmL in a microplate format. The absorbance measured with the spectrometer matches the known absorbance of the filter measured at the intended wavelength.
The absorbance at a wavelength of 280 nm was 15. By measuring the absorbance of several solutions of known concentration we are able to prepare a graph that can be used to determine concentrations of unknowns. Using the values you obtained for A c and l plug them into the equation ɛ Alc.
17 The rate constant of a particular reaction can be determined by measuring the UVVis absorbance spectrum at. A Log 10 I 0 I. This wavelength is known as λ max and is most sensitive to the changes in concentration.
Spectrophotometry uses photometers known as spectrophotometers that can measure the intensity of a light beam at different wavelengthsAlthough spectrophotometry is most commonly applied to. Review the instructions for using the spectrophotometer if you have forgotten. CV A 1 cmε 3 The first step in this analysis will be to determine the wavelength of maximum absorbance for crystal violet and the corresponding molar absorption coefficient at that wavelength.
Therefore The absorbance value observed at high concentrations is lower than what it should be. Most pharmaceuticals natural products clinical samples and petroleum products have fluorescent absorbance. It is recommended to test absorbance accuracy at the same wavelength using several filters with different absorbance levels.
This experiment was performed to determine the factors that positively influence enzyme reaction rates in cellular activities since some enzymes seem to be more effective than others. Figure 1 shows the absorbance increasing as the wavelength is increased until approximately 650 nm is reached. What is the concentration.
Hover the cursor over the maximum absorbance peak and record the corresponding wavelength x-axis and absorbance y-axis. When using a spectrophotometer to determine concentration of a sample solution of unknown concentration by UVVIS spectroscopy a calibration line must first be created. The intensity of this emitted light is monitored to quantify the analyte concentration.
This is not difficult because absorbance is proportional to dye concentration via Beers Law. T II 0 and T 100 T. All we need to do is determine this relationship.
Look at the images above. Absorbance of buffer reagents does not overlap with absorbance of the analyte. Using a standard curve.
Determine concentration using the Beer-Lambert Law. This is reflected in the asymptotic flattening of a standard curve observed at high concentrations. The calibration plot is like taking a vertical slice through the all the absorbance spectra at the specific wavelength 410nm.
Using your absorbance readings and the molar concentrations construct a Beers Law plot plot the molar concentrations of your known solutions on the x-axis and the absorbance data on the y-axis. Choosing Your Wavelength. A 2 - log 10 T.
Repeat steps 5-11 for the remaining. Learn how absorbance is measured on our absorbance microplate readers with our featured app notes. Using a cuvette with a length of 1 cm you measured the absorbance of a solution with a concentration of 005 molL.
By using a specific wavelength analyte atoms are excited and then emit light signal fluorescence. Spectrophotometry Page 2 of 10 solution Transmittance or absorbed Absorbance by the solution is measured by a light meter. Using optical fibers as a transmission element of spectrum of burning gases it is possible to determine a chemical composition of a fuel temperature of gases and air-fuel ratio.
This is done by measuring the light absorption of several standard solutions of different known concentrations at a predefined fixed wavelength. Relationship is best determined using a wavelength of light in a region of the visible spectrum where the maximum absorbance is observed. The wavelength 410nm was a very good choice for the calibration plot but how do you know which wavelengt is the best wavelength based on the absorbance spectrum.
The equation that allows one to calculate absorbance from transmittance is. 100 Absorbance 0 Transmittance While a spectrophotometer can display measurements as either transmittance or absorbance in biological applications we are usually interested in the absorbance of a given sample. Catecholase enzyme activity was measured through its absorption rate in a spectrophotometer using light with a wavelength of 540 nm.
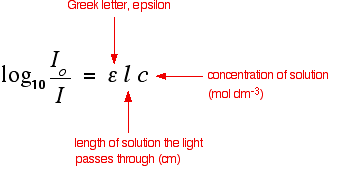
Using Uv Visible Absorption Spectra

Methylene Blue Blue Shift How To Correctly Measure The Mb Concentration

Resolution And Stray Light Uv Vis Spectroscopy Faq
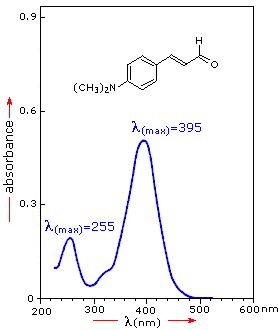
Uv Visible Spectroscopy
Determination Of Absorbance Of Kmno4
Beer S Law Lab Explained Absorbance Vs Concentration Schoolworkhelper

Absorbance Detection Surgical Instruments Research Instruments Laboratory Equipment Wpi
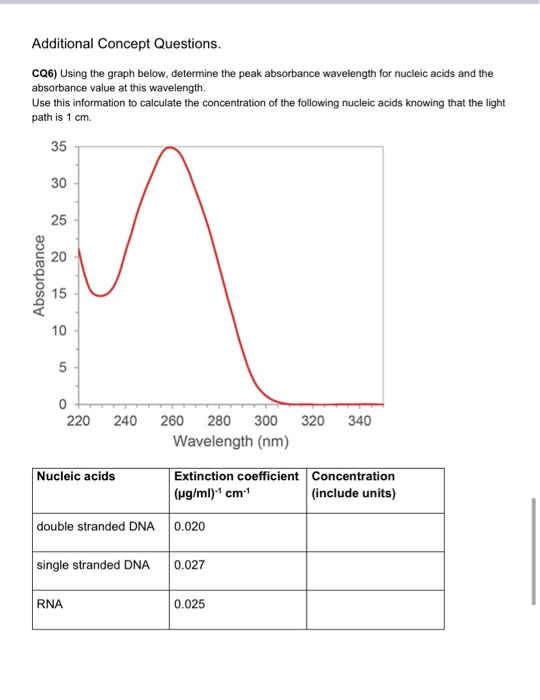
Solved Additional Concept Questions Cq6 Using The Graph Chegg Com

